Neuralink, Elon Musk’s brain‑computer interface venture, is entering a new phase: the company has reportedlysubmitted human patient data to a peer‑reviewed journal, marking a milestone in its journey from ambitious concept to clinical legitimacy. While many of Musk’s projects tend to capture headlines more for their visionary scope than their near‑term deliverables, the data now under review appears to provide concrete findings. This article investigates what is known, what remains uncertain, and what implications this could have for Neuralink, for neuroscience, and for regulatory oversight.

What Is Neuralink and Where It Stands
Founded in 2016, Neuralink aims to build implantable brain‑chip interfaces (BCIs) that allow communication between neurons in the brain and external devices. Its stated goals include helping people with severe paralysis control computers or prosthetic devices, restoring vision to those who have lost it, and eventually more ambitious applications such as direct thought‑to‑text capabilities.

Neuralink has faced the usual scientific, ethical, and regulatory challenges: safety of implants, durability of the electrode threads, reactions of brain tissue, risk of infection, and questions about long‑term performance. Moreover, in the past, it has been criticized for lack of transparency and for delays in achieving some earlier promised deliverables.

The Submitted Data: What We Know
In October 2025, reports indicate that Neuralink has submitted a scientific manuscript to The New England Journal of Medicine (or another high‑prestige peer‑review journal) describing results from human patients implanted with its device. Importantly, according to statements from Michael Lawton, a clinical trial site leader involved with Neuralink, the paper includes safety data from the first three patients implanted.
Some additional details gleaned from public reporting:
Neuralink has implanted devices into at least three patients, though at other times broader numbers (e.g. twelve) are mentioned as having been implanted.
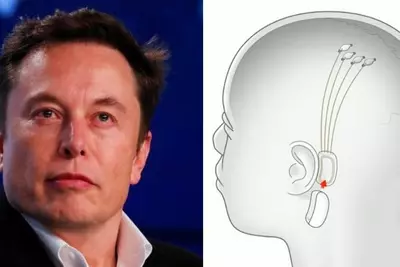
These implants are of Neuralink’s brain‑chips (often referred to as “N1” or “Telepathy”) intended to enable severely disabled individuals (for example, those with quadriplegia or ALS) to control digital devices by thought alone.
The data includes findings related to neuron spike detection, which is the chip’s ability to detect electrical activity from neurons. Early reports suggest that this detection is “promising”.
Safety is also a focus: whether the implantation process, the device materials, signal stability, inflammation, degradation of performance over time, or tissue reactions present significant risk. The submitted data reportedly includes some safety assessments.
Potential Significance
Assuming the submitted data holds up to peer review, several major implications follow:
Proof of Principle in HumansUp to now, many demonstrations have been in animal models—monkeys, pigs, etc. If Neuralink can show that its device works in human patients safely and reliably, that advances the field considerably. It moves from speculative to applied.
Validation of Signal Detection and Usability
Demonstrating neuron spike detection means that the chip is actually “reading” neural signals meaningfully. For users with severe motor disabilities, being able to send signals to control a cursor, a device, or other assistive technology via thought depends on the fidelity of those signals.
Regulatory MomentumHaving robust human‑data that includes safety metrics is critical for regulatory approval. Getting the FDA’s “Breakthrough Device” designation (for some of Neuralink’s products, such as “Blindsight”) has helped accelerate the process. Such a submission could further support regulatory paths for broader trials, approvals for specific indications, and eventual consumer applications.
Investor Confidence and FundingShowing real human data helps reassure investors, stakeholders, and potential partners that Neuralink’s technology is not purely theoretical. The company has raised large sums (hundreds of millions of USD) and has been valued in the billions. Demonstrated results can influence its valuation, partnerships, and speed of rollout.

Key Uncertainties and Risks
Despite the promising signs, there are several caveats and areas where information is still thin, which bear scrutiny:
Sample Size and Duration
With only three patients reported in the submitted paper, statistical power is limited. Longer follow‑ups are needed to see how stable the implant performance remains, how adverse events (if any) emerge over months or years, and whether signal fidelity declines.
Specifics of Function
Detecting neural spikes is one thing; translating those into useful, high‑bandwidth commands (typing, controlling a robotic limb, speech restoration, etc.) is much more complex. It’s not clear how many degrees of freedom the system supports, how much lag or error there is, or how intuitive control will be.

Long‑term SafetyIssues like brain tissue response, scarring, infection risk, device component failure, drift of electrodes, battery life (if applicable), and risk of surgical complications remain. The human brain is a sensitive environment.
Ethical & Privacy ConcernsAs implants become more capable of reading complex cognitive states, privacy, consent, data security, and potential misuse become more pressing. There is also concern about equality of access – whether such devices will be expensive and restricted to privileged populations.
Regulatory HurdlesEven with “Breakthrough Device” status, full clearance for medical device use (especially surgical implants) is strict. The FDA and other regulatory agencies will demand robust evidence, careful trial design, long‑term data, and clarity on manufacturing standards.
What Has Been Criticized in the Past?
To understand where the promise may falter or what obstacles remain, it’s informative to look at past criticisms:
Animal Testing: Neuralink has been cited by the FDA for issues with its animal research labs—some practices or conditions were found deficient in oversight or documentation. While the FDA noted concerns, they did not, in those cases, find violations severe enough to require formal regulatory action.

Overpromising: Some observers note that Musk and Neuralink have often announced aggressive timelines (e.g., human trials “six months away”) and ambitious goals (e.g., restoring full movement, enabling telepathy‑like control) which may exceed what is realistically possible in near term.
Transparency and Independent Verification: Until now, much of what we know has come from press statements, Musk’s posts, or secondary reports. Peer‑reviewed publication of human data is a step forward, but independent replication, open data (for oversight), and detailed safety findings will be critical.
What Submitted Data Suggests So Far
From what is publicly reported, the submitted data suggests:
The first three human implants have been performed, and the patients are recovering well (no serious immediate adverse events reported). Neuralink’s device is detecting neural activity (“spike detection”), which is essential for any higher‑level functionality (e.g. translating neural signals to digital commands). This suggests a workable interface between neurons and electronics, at least in terms of signal capture.
The company is collecting safety data, and presumably monitoring patients for signs of inflammation, infection, device drift, etc. While details are not yet public in full, the submission implies that some short‑term safety benchmarks have passed.

What’s Next: What to Watch For
Moving forward, several developments will be key in assessing how much promise the submitted data actually represents.
Peer Review and PublicationWill the manuscript pass peer review? What journal will publish it? Full access to the data (methods, limitations, adverse events) will be necessary for the scientific community to evaluate its claims.
Long‑Term Follow‑UpHow do patient outcomes evolve over time: do signal quality, patient interface usability, safety, and device reliability remain high after months, years?

Functional OutcomesNot just detection, but meaningful control: How well can patients perform tasks? How intuitive is control? What are the error rates, latency, bandwidth?
Expansion of Trial SizeIncreasing number of participants, more diverse subjects (ages, severity, comorbidities), more independent study sites to rule out bias.
Regulatory Approvals / Commercialization PathWill FDA and other regulatory bodies approve for specific indications? When might such devices become commercially available, and under what constraints?
Ethical, Social, and Legal OversightAs implants become more capable, society will need to address who gets access, how data is handled, how to avoid exploitation or harm. Issues of privacy, autonomy, and consent are central.

Potential Impacts and Controversies
If Neuralink’s human data holds up, implications are broad:
Medical Rehabilitation: Patients with paralysis, spinal cord injuries, ALS, or stroke could gain new ability to communicate, control prosthetics or devices, improving quality of life significantly.
Assistive Technology Revolution: BCIs could transform interfaces: e.g. controlling computers, phones, or even environments through thought. This may reshape how people with disabilities interact with the world.

Vision Restoration: With devices like “Blindsight” aimed at restoring vision in cases where optic nerves are damaged but visual cortex remains intact, a subset of blind people might regain visual perception.
Speech / Thought‑to‑Text: If brain signals can be decoded into speech or text reliably, this could open new frontiers for those who cannot speak, but also raise debates about privacy, mental overreach, and cognitive liberty.
Scientific Knowledge of the Brain: Better data will deepen understanding of brain signal decoding, neural plasticity, safety of implants, and the interface between machines and biological tissue.
However, with these potential benefits comes controversy:
Risks associated with brain surgery.
Potential inequities: who gets access, who bears cost, liability issues if devices fail.
Risks of misuse: privacy of neural data, potential for surveillance, thoughts being decoded without consent.
Assessment: How Much Promise?
On balance, the submission of human data is a strong signal that Neuralink is moving beyond hype toward empirical scrutiny. The promise is real—as in, this isn’t purely futuristic speculation anymore. But it is early. Much depends on the details that are not yet public (e.g. how easy the device is to use, how stable performance is over time, side‑effects, cost, etc.).
If I were to grade current status:
Scientific plausibility: High — detecting neural spikes, successfully implanting devices, reports of patient recovery, etc.
Clinical maturity: Moderate but early — small sample size, short follow up, some unknowns.
Commercial viability: Low to moderate in short term — many regulatory and manufacturing challenges ahead.
Social / ethical readiness: Mixed — public enthusiasm, but oversight and frameworks trailing behind.

Conclusion
Elon Musk’s Neuralink appears to have submitted its first batch of human patient data for peer review, including findings of neuron spike detection in several implanted individuals. This is a milestone. It indicates that Neuralink is stepping up from animal studies and conceptual presentations to data that invites independent examination. Such transparency, if maintained, could accelerate progress not just for Neuralink but for the field of brain‑computer interfaces generally.
Yet much remains to be seen. How well the implants function over time, how usable they are in everyday life, how safe they are in longer follow‑ups, and how society addresses the ethical and regulatory implications will determine whether this promise becomes reality.
For now, the data submitted provides a cause for cautious optimism: the technology is no longer entirely speculative. The next months and years will tell whether Neuralink can deliver on its visionary promises — restoring function, restoring voices, perhaps even restoring vision — in a safe, ethical, and accessible way.
News
New Colossus: The World’s Largest AI Datacenter Isn’t What It Seems
In a quiet corner of the American Midwest, a sprawling facility has been generating whispers among tech insiders, policy analysts,…
Kayleigh McEnany: This is Sending the World a Message
Kayleigh McEnany, former White House Press Secretary and political commentator, has long been recognized for her unflinching communication style and…
Candace Says Thiel, Musk, Altman NOT HUMAN
In a statement that has sparked widespread discussion across social media and news platforms, conservative commentator Candace Owens recently claimed…
Judge Pirro Reveals HARDEST Part of Job as US Attorney
Judge Jeanine Pirro is a household name in American media and law, known for her sharp wit, commanding presence, and…
Harris Faulkner: This Could Potentially EXPLODE
In the constantly shifting landscape of American media, few figures have sparked as much debate, admiration, and scrutiny as Harris…
Kaido is CRASHING OUT After Salish DUMPS Him For Ferran (Nobody Saw This Coming)
When word broke that Salish Matter had dumped Kaido and seemingly moved on with Ferran, the internet didn’t just react…
End of content
No more pages to load












